
Advancing HPAPI Safety
Streamline SMEPAC containment verification
Nanozen Pharma DustCount provides unparalleled real-time data on contaminant levels, enabling pharmaceutical manufacturers to proactively mitigate safety risks and achieve a new standard for protecting workers, products and the environment.
SMEPAC traditional
HPAPI with unknown safety profile
Highly potent active pharmaceutical ingredients (HPAPIs) are at the forefront of modern therapeutics, offering powerful solutions for conditions such as cancer and autoimmune diseases. However, their potency comes with a unique challenge—addressing potential toxicities when manufacturing, particularly when their safety profiles are not fully characterized. How can CMOs and CDMOs safeguard their workforce, prevent cross contamination, ensure product quality, and protect the environment?
 Moreover, in the context of handling highly potent active pharmaceutical ingredients (HPAPIs), assessing occupational exposure solely based on time-weighted average (TWA) values (determined by the traditional pump/filter method), often falls short of addressing the risks posed by short-term exposure spikes. These transient peaks in exposure can have significant implications for worker safety, particularly for substances with acute toxicity or sensitizing properties. The challenge is further compounded by the unknown safety profiles of many HPAPIs and the relentless pressure to maintain efficient production timelines, making the role of CDMOs increasingly complex and demanding.
Moreover, in the context of handling highly potent active pharmaceutical ingredients (HPAPIs), assessing occupational exposure solely based on time-weighted average (TWA) values (determined by the traditional pump/filter method), often falls short of addressing the risks posed by short-term exposure spikes. These transient peaks in exposure can have significant implications for worker safety, particularly for substances with acute toxicity or sensitizing properties. The challenge is further compounded by the unknown safety profiles of many HPAPIs and the relentless pressure to maintain efficient production timelines, making the role of CDMOs increasingly complex and demanding.
The current containment verification (SMEPAC) method for measuring HPAPIs in the air involves using a pump to draw air and collect particles onto a filter, which is then sent to an analytical lab for analysis. However, this approach introduces significant time delays, making it challenging to promptly identify equipment leaks. As a result, repeated tests and additional resources are often needed to pinpoint potential exposure risks, creating uncertainties in ensuring safe production.
SMEPAC Pre-test
Real-Time Monitoring as a Solution
Real-time monitoring technologies, like the Nanozen Pharma DustCount, offer a significant advancement by providing instantaneous data on contaminant performance levels. This enables immediate detection of leaks, allowing for proactive intervention and preventing issues from escalating, which is an ideal tool to conduct SMEPAC pre-test to save money and time.
MICROSIZE Case Study
Case studies at MICROSIZE, a world leader at particle reduction OEB 4 CDMO facility in Philadelphia.
Real-time assisted SMEPAC pre-test
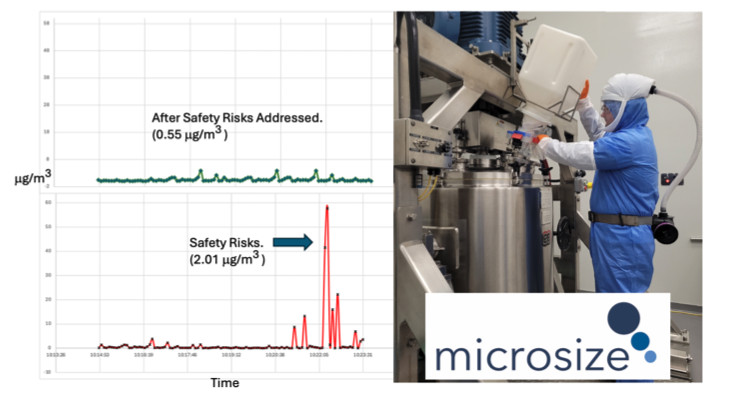
Microsize has joined the ranks of leading global pharmaceutical companies committed to minimizing short-term exposure spikes. Abiraterone acetate, a high potency active pharmaceutical ingredient HPAPI used in prostate cancer treatment, with an OEL of 7 μg/m3 was selected as the test material for this study. Utilizing Nanozen’s real-time API monitor, specifically calibrated to abiraterone acetate, we assessed personal exposure levels and potential cross-contamination risks following SMEPAC guidelines. Three common unit operations including dispensing, blending, and dry powder milling all of which had been outfitted for < 1 μg/m3 containment performance utilizing soft-wall isolation, continuous liner systems and using active/passive mating valve systems.
By utilizing Nanozen technology as SMEPAC pre-test tools, we successfully detected and captured a brief exposure spike (<15 seconds safety risks) that would have been overlooked or diluted with conventional method. This data enabled precise root cause identification and the implementation of a 'right the first time' corrective action, ensuring effective resolution and establishing best practices as standard procedure." Microsize's CDMO facility showcased its ability to confidently and consistently manage OEB 4 products by implementing process improvements and integrating real-time, API-specific monitoring systems.
Conclusion:
ISPE SMEPAC (Standardized Measurement for Equipment Particle Airborne Concentration) is an industry benchmark for assessing containment performance. The guideline has provided pharmaceutical facilities with valuable insights into implementing HPAPI protection. However, its non-real-time approach has limited efficiency in safeguarding resources and streamlining tests. Nanozen integrates SMEPAC principles with real-time monitoring to conduct pre-test to isolate leaks, addressing non-real-time testing challenges by not only eliminate short-term exposure spikes, but also minimizing environmental contamination through early leak detection.
By preventing API leakage into surrounding environments, Nanozen promotes sustainable manufacturing practices that align with global environmental standards. Empowering pharmaceutical manufacturers to enhance worker safety and ensure regulatory compliance, Nanozen is your partner in creating a safer, more sustainable future. Contact us today to schedule a demonstration or receive a free personalized consultation tailored to your operational needs.